Case Series - (2020) Volume 2, Issue 3
Introduction: Lutetium-177-PSMA-617 (177Lu-PSMA) is used as the last treatment option in metastatic castrate resistant prostate cancer (mCRPC). We present two cases of mCRPC with bone involvement to see the response of 177Lu-PSMA as an alternative treatment option.
Case presentations: We present two cases, diagnosed as mCRPC with bone involvement, having suboptimal labelling and binding of the tracer and its effect on bone marrow. The two patients had 2-4 cycles of 177Lu-PSMA with progressive disease despite chemotherapy and novel androgen therapy. The response of treatment was determined based on changes in radiological findings, biochemical and post-therapeutic scan. The last post therapy SPECT CT 99mTc-PSMA scans in both patients showed diffuse homogenous tracer distribution in the bone marrow with no suggestion of receptor avid disease. In both patients the PSA level decreased. Side effects seen were pancytopenia and mild increase in creatinine and urea.
Conclusion: The main benefit of 177Lu-PSMA is to offer an alternative therapeutic option for patient with disease progression despite first line of treatment. We advocate a repeat scan is required after every treatment session with 177Lu-PSMA to ensure adequate radioisotope uptake and to assess for therapeutic response. A patient specific dosimetric approach should be applied before therapy to prevent organ toxicity.
Nuclear medicine•Prostate specific antigen•Bone lesion•Androgen resistant•PSMA
Prostate Cancer (PC) is one of the most prevalent cancers worldwide. Progression to androgen-independent status is the main cause of death in patients with prostate cancer [1]. Most deaths are related to metastatic disease, which results from any combination of blood, lymphatic, or local spread. Bone metastases, a major cause of morbidity and mortality in patients with castration-resistant prostate cancer, are associated with pain, pathological fractures, spinal cord compression, and decreased survival [2].
The PSMA is a transmembrane protein that is over-expressed in advanced prostate cancer [3]. PSMA is highly expressed on prostate epithelial cells and strongly upregulated in prostate cancer. PSMA expression levels are directly correlated to androgen independence, metastasis, and progression [4]; thus, it is an attractive target for the diagnosis and therapy of metastasized prostate cancer.
A novel theragnostic drug, 177Lu-PSMA, which is a DOTA-derivative of the Glu–urea–Lys motif, it is an example of PSMA-guided therapies, which together acts as a means of transporting destructive radiation to the tumor site without exposing the healthy cells [5,6].
We present a case series of two patients of mCRPC who underwent 177Lu-PSMA therapy with suboptimal labelling and binding of the tracer with poor outcomes.
Case 1
69 years old male patient was initially diagnosed as metastatic prostate cancer with bone involvement in 2017. He has past history of Diabetes Mellitus, Hypertension and Cerebrovascular accident in 2015. His ECOG performance status was 1. The initial PSA value during diagnosis was 854 mcg/L (normal range=<4.1 mcg/L). He received treatment with dual androgen blockades; Leuprolide, Bicalutamide and Zometa. Then started on 177LuPSMA with a total of 4 cycles. After the first cycle his PSA dropped to 36 mcg/L. Three months later after the third cycle of treatment PSA was 8.6 mcg/L. Treatment was completed in May of 2018 with PSA value was within normal range.
In March 2019 patient presented with significant low back pain and a PSA value of 130 mcg/L. Treatment with Zoladex, Abiraterone and Denosumab was initially commenced. 68Ga-PET CT was performed during that time and it showed metastatic lesion within bone with increased intensity compared to a scan in July 2018. 99mTc-PSMA whole-body with SPECT-CT, showed widespread receptor avid bony disease. Diagnosis of metastatic castrate resistant prostate cancer with disease progression was made despite being on antiandrogen therapy. Treatment was changed to Abiraterone Acetate, Prednisolone, Zoladex for 3 month and Denosumab once a month. Therapy with 177Lu-PSMA was recommended.
Prior to initial therapy, laboratory investigation demonstrated PSA level of 214 mcg/L, a free PSA of 47.600 mcg/L (normal range= 0.084- 0.870 mcg/L) and PSA% of 22%. A complete blood count showed white blood cell (WBC) count was 6.7 × 109/L (normal range= 4.5-11 × 109/L), hemoglobin (Hb) was 10.7 g/dl (normal range=12.6-17 g/dl) and platelet count was 146 × 109/L (normal range= 140-400 × 109/L). Mag-3 nuclear scan for renal function demonstrated adequate renal function and a creatinine level 100 micromol/L (normal range= 62-106 micromol/L). Before radiotracer can be started patient was hydrated well with IV fluids.
Post therapy SPECT CT scan after the first cycle of 177Lu-PSMA demonstrated multiple foci of increased tracer uptake throughout the bony skeleton. The most avid uptake was seen in the left orbital margin, L5, left SI joint, and left hip joint. Laboratory investigation post therapy revealed hypokalemia of 2.6 mmol/L (normal range=3.2-5.6 mmol/L), rest were within baseline levels. The SPECT CT scan after the second cycle of 177Lu-PSMA, demonstrated good response to treatment. Laboratory values showed a drop in WBC of 3.6 ×109/L and platelet count of 85 × 109/L. Before commencement of third cycle PSA level was 181 mcg/L.
After third cycle the response assessment on post therapy SPECT CT scan could not be appreciated because of diffuse marrow uptake seen on the scan without delineating any focal receptor avid lesions seen on previous post therapy scans. Therapy with 177Lu-PSMA showed a significant decrease in PSA levels, with reduction of 71% (Figure 1).
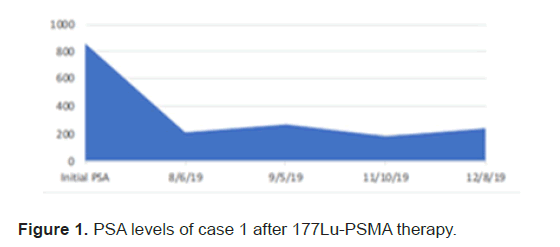
Figure 1: PSA levels of case 1 after 177Lu-PSMA therapy.
Each cycle of therapy was given one month apart. No complications or side effects during therapy sessions. Clinically patient’s symptoms were controlled with adequate pain relievers. The end of the therapy there was a significant drop of 70%, 25% and 60% in platelet count, Hb & WBC, respectively (Figure 2). The PSA level at the end of treatment was 241 mcg/L, free PSA of 28.900 mcg/L and PSA% of 12%. The last post therapy SPECTCT 177Lu-PSMA scan showed diffuse homogenous tracer distribution seen in the bone marrow, suggestive of bone marrow uptake and no receptor avid disease (Figure 3A), as compared to baseline scan (Figure 3B).
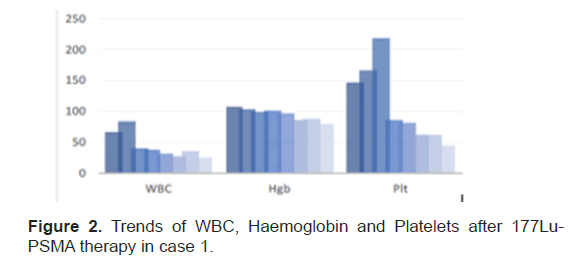
Figure 2: Trends of WBC, Haemoglobin and Platelets after 177Lu- PSMA therapy in case 1.
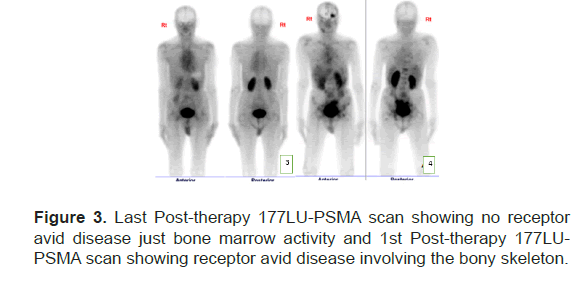
Figure 3: Last Post-therapy 177LU-PSMA scan showing no receptor avid disease just bone marrow activity and 1st Post-therapy 177LUPSMA scan showing receptor avid disease involving the bony skeleton.
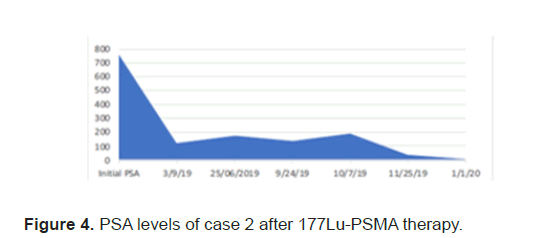
Figure 4: PSA levels of case 2 after 177Lu-PSMA therapy.
Case 2
46 years old male patient initially presented a year ago with significant left hip pain controlled with Morphine. Investigations were done showed a PSA level of 752 mcg/L and imaging with CT and nuclear bone scan demonstrated widespread bony metastasis with foci seen throughout the cranium, spine, rib cage, upper and lower extremities, manubrium, sternum, and pelvic bones. Complete blood count revealed pancytopenia from bone marrow involvement. The diagnosis of prostate cancer was made despite no biopsy at that time as he was ill and quite symptomatic from the bone metastases. He was given Leuprorelin and abiraterone/ prednisolone. He also received radiotherapy to symptomatic T7, L5 and left hip. He responded to the treatment and his PSA dropped to 6.1 mcg/L after 2 months of initial diagnosis. Patient was on Morphine for his low back pain and was able to walk with ECOG performance status of 0.
After 6 months of completion of initial management the patient presented with significant severe bilateral leg pain up to mid-calf with limited mobility. His PSA level rose up to 34.8 mcg/L and imaging with CT demonstrated progression of extensive bony metastasis with newly developed compression fractures of T7 and L5 vertebral bodies. There was slight retro bulging of the L5 vertebral body, which resulted in mild cord compression. The SPECT CT bone scan revealed significant increased in the number of sites of osteoblastic activities in the axial and appendicular skeleton; throughout the spine, bilateral ribs, proximal humeri, frontal bone, scapulae, sternum, pelvis, right proximal femur. Patient was started chemotherapy with Docetaxel and prednisolone.
The PSA levels progressed up to 189 mcg/L and patient was planned for 177Lu-PSMA therapy. 177Lu-PSMA which revealed widespread PSMA receptor avid disease in the axial and proximal appendicular skeleton. Complete blood count before therapy showed WBC 8.7 × 109/L, Hb 10.5 g/ dl and Platelet 176 × 109/L. Renal function was normal with creatinine level of 60 micromol/L and adequate function without outflow obstruction seen in 99mTc- Mag-3 scan. Chemotherapy was stopped due to progression of disease after a total 5 cycles. Patient completed 2 cycles of 177Lu-PSMA with response seen in SPCET CT 177Lu-PSMA post therapy scan. The PSA level had a reduction of 92% (Figure 4). After 2 cycles patient developed pancytopenia requiring transfusions (figure 5). After the 3rd therapy dose the patient developed myelosuppression with pancytopenia and required admission for transfusion. The post therapy 99mTc-PSMA with SPECT CT scan showed diffuse homogenous tracer distribution in the bone marrow with no suggestive of bone marrow uptake and no receptor avid disease (Figure 6A), as compared to the baseline scan (Figure 6B). Therapy was stopped as patient developed severe sepsis requiring admission under intensive care.
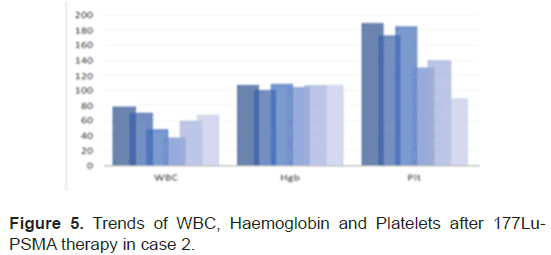
Figure 5: Trends of WBC, Haemoglobin and Platelets after 177Lu-PSMA therapy in case 2.
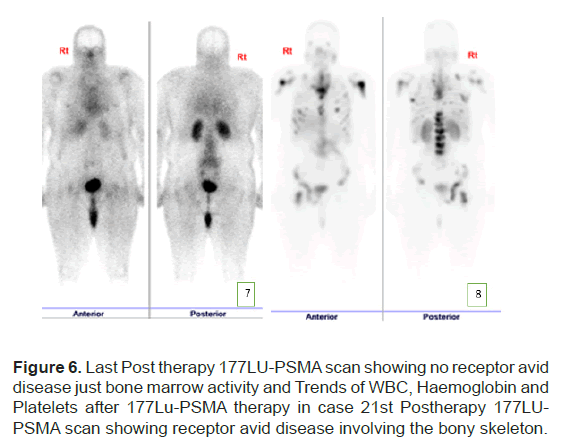
Figure 6: Last Post therapy 177LU-PSMA scan showing no receptor avid disease just bone marrow activity and Trends of WBC, Haemoglobin and Platelets after 177Lu-PSMA therapy in case 21st Postherapy 177LUPSMA scan showing receptor avid disease involving the bony skeleton.
We report two cases of metastatic prostate cancer with metastasis to bone that were pre-treated with chemotherapy and anti-hormonal therapy with initial good response and stable disease on imaging. But after one year there was an increased in PSA level and progression of mCRPC. Both patients underwent 177Lu-PSMA therapy with suboptimal response.
The last post therapy SPECT CT 99mTc-PSMA scans in both patients showed diffuse homogenous tracer distribution in the bone marrow with no suggestion of receptor avid disease. All of which raises the question of suboptimal uptake of radioisotope targeting the tumor cells, therefore narrowing the therapeutic window. The only reason we could contribute to this was suboptimal labelling and poor binding of the tracer. The reasons for poor labelling are still unclear as they may be related to the peptide/ buffer tagging to the Lu177, the transportation conditions that may affect the labelling efficiency, and/or the solutions used for the quality control that can mislead to pass QC results. We attribute bone marrow suppression to free 177Lu, although the QC was more than 95% for both 177Lu-PSMA therapy doses. Further studies are required to confirm the cause of pancytopaenia in these two patients.
Wright et al. have demonstrated that while PSMA is expressed at low levels in normal human prostate epithelium, it is overexpressed up to 1000 times higher in almost all prostate cancer cells than in normal prostate cells [7]. It was observed that the density of expression of this transmembrane receptor on prostate cancer cells further increases depending on hormone-resistant prostate cancers [8].
PSMA is not entirely prostate specific and is expressed in cells of small intestine, proximal renal tubules and salivary glands [8]. Although the expression of PSMA on these cells is significantly reduced when compared to prostate cancer cells, there is a radiation dose that can be delivered to these target organs [8]. This has an impact on the benefit/ risk profile and determination of the safe dose of radiotherapy that can be delivered without causing significant radiation damage to non-target organs. This is an important consideration for our patient as it is possible that PSMA-targeted therapy will be affecting other tissues that are positive for PMSA receptors especially the kidney. In fact, patients with mild or moderate renal impairment may be at greater risk of toxicity. Therefore, it is suggested to perform more frequent assessments of renal function in patients with mild to moderate renal impairment.
Emmett et al. reported that 177Lu-PSMA has gained popularity as the therapeutic radionuclide of choice due to its physical properties [8]. 177Lu-PSMA is a low-energy β-particle emitter which limits the distance β-particles can travel through tissue. The shorter β-range of 177Lu- PSMA provides better irradiation of small tumors, compared to longer β-particles. The γ-emission from 177Lu allows for ex vivo imaging and consequently the collection of information pertaining to tumor localization and size, which has the advantage of follow-up of 177Lu-PSMA therapy. It has relatively long physical half-life of 6.73 days, which allows for the delivery of high activity 177Lu-PSMA to prostate cancer cells [8]. 177Lu- PSMA is an easily be administered without significant symptoms at the time of injection. The main safety issues are standard radiation safety precautions that are inherent in all intravenously injected, renal excreted radionuclide therapies [8,9].
In the TROPIC, phase III clinical trial, the number of men reduction in serum PSA levels from 30% to 70%, which is highly comparable to the PSA response rates achieved by chemotherapy agents used in mCRPC, like Cabazitaxel and Docetaxel [10]. Baum et al. included 56 men; 80% of all men had reduction of PSA levels after therapy [11]. Patients were receiving up to five treatments of 177Lu-PSMA at 6-weekly intervals appeared to have possible survival benefits. This study found that with a follow-up period of 28 months, 12 patients died (21.4%). Survival after 28 months was 78.6%. Median progression-free survival was 13.7 months [11]. Tagawa et al. published the results of a Phase II clinical trial that demonstrated 177Lu-PSMA resulted in declines in PSA among patients with mCRPC [12]. In a follow-up analysis, they reported a better response, including increased survival, but with higher toxicity with increased dose [12]. Additionally, a large decline in Circulating Tumor Cells (CTCs), which is an important biomarkers for the follow up treatment of advanced prostate cancer [13].
33% of those men treated to date show progressive disease despite treatment [8]. These are likely due to a variety of factors. One important factor is the tumor cells uniformly express a high density of the PSMA receptor in the targeted tissue. Because of heterogeneity of PSMA receptor activity within the tumor population may mean that some sites will not respond to treatment with 177Lu-PSMA, which will manifest as disease progression, and in a rising of the PSA [8]. Bone metastases appeared to respond less well than visceral or lymph nodal disease to treatment with 177Lu-PSMA [11].
Overall, toxicities related to 177Lu-PSMA therapy have been of low grade and manageable with close monitoring. Hematological toxicity is the most commonly reported serious side effect related to 177Lu-PSMA therapy that also deserves a close monitoring and the follow-up of local specific clinical practices guidelines. Ferdinandus et al. reported that 40 patients identified low platelet level and the need for pain relief as the most significant predictor of poor response to 177Lu-PSMA [14]. In men with significant bone metastases, up to 10%-25% of men had a Grade 1-2 reduction in hemoglobin or platelets that should be clinically manageable [8]. Because of the longer particle range of 177Lu-PSMA, compared to alpha emitters such as radium 223, it is likely that 177Lu- PSMA will have a higher radiation dose to surrounding marrow in men with extensive metastatic bone disease, than alpha emitter treatment options [15]. Another concern about toxicities was kidneys due to the mechanism of excretion it is an anticipated long-term complication [16]. Safety and efficacy of targeted radionuclide therapies can be improved with patientspecific dosimetry, which can act as an early indicator of organ toxicity [8].
All currently published studies about 177Lu-PSMA therapy in prostate cancer are retrospective, mostly single arm, and involve a variety of treatment regimens, both in terms of dose given (ranging from 3.5 to 8.0 Gbq/injection of 177Lu-PSMA) and the number of doses administered (ranges from a single injection up to 4-6 injections 6 weeks apart) [8]. Currently there are no long-term prospective Randomized Controlled Trials (RCTs) available for the evaluation of PSMA-targeted radioligand therapy on survival benefits compared with approved standard therapies for advanced prostate cancer such as abiraterone, enzalutamide, radium- 223-dichloride, docetaxel or cabazitaxel.
177Lu-PSMA therapy for progressive mCRPC is safe, effective, welltolerated, and has a considerable effect on PSA levels. The main objective of the therapy is to minimize toxicity in patients whose disease had progressed despite all standard treatments. The factors contributing to our therapy related side-effect and outcomes of treatment are suboptimal and our experienced in our cohort of patients remains unanswered. Therefore, a patient specific dosimetric approach should be applied before therapy to prevent organ toxicity.
Author 1; Contributed in case presentation and writing of manuscript
Author 2; Contributed in manuscript writing and structured manuscript according to journal format
Author 3; Contributed in preparation of the case and provided images
Author 4; Contributed in editing the manuscript and provided images
Author 5; Contributed in prepared the case
Author 6; Contributed in preparing the case
Author 7; Contributed in preparing the case
Author 8; Contributed in preparing the case
Author 9; Contributed in editing and proofreading the manuscript
Author 10; Contributed in preparing the case and in editing the manuscript
Author 11; Contributed in preparing the case, editing the manuscript and provided images
Citation: Sheebani SA, et al. 177Lu-PSMA Therapy an Alternative Therapy for Metastatic Castrate Resistant Prostate Cancer with Suboptimal Response: Are we ready for it. Eur J Clin Oncol, 2020, 2(2), 001-004
Received: 23-Sep-2020 Published: 15-Nov-2020
Copyright: © 2020 Altinoz A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.